Research
Monitoring and manipulating the immune-microbiota interactions
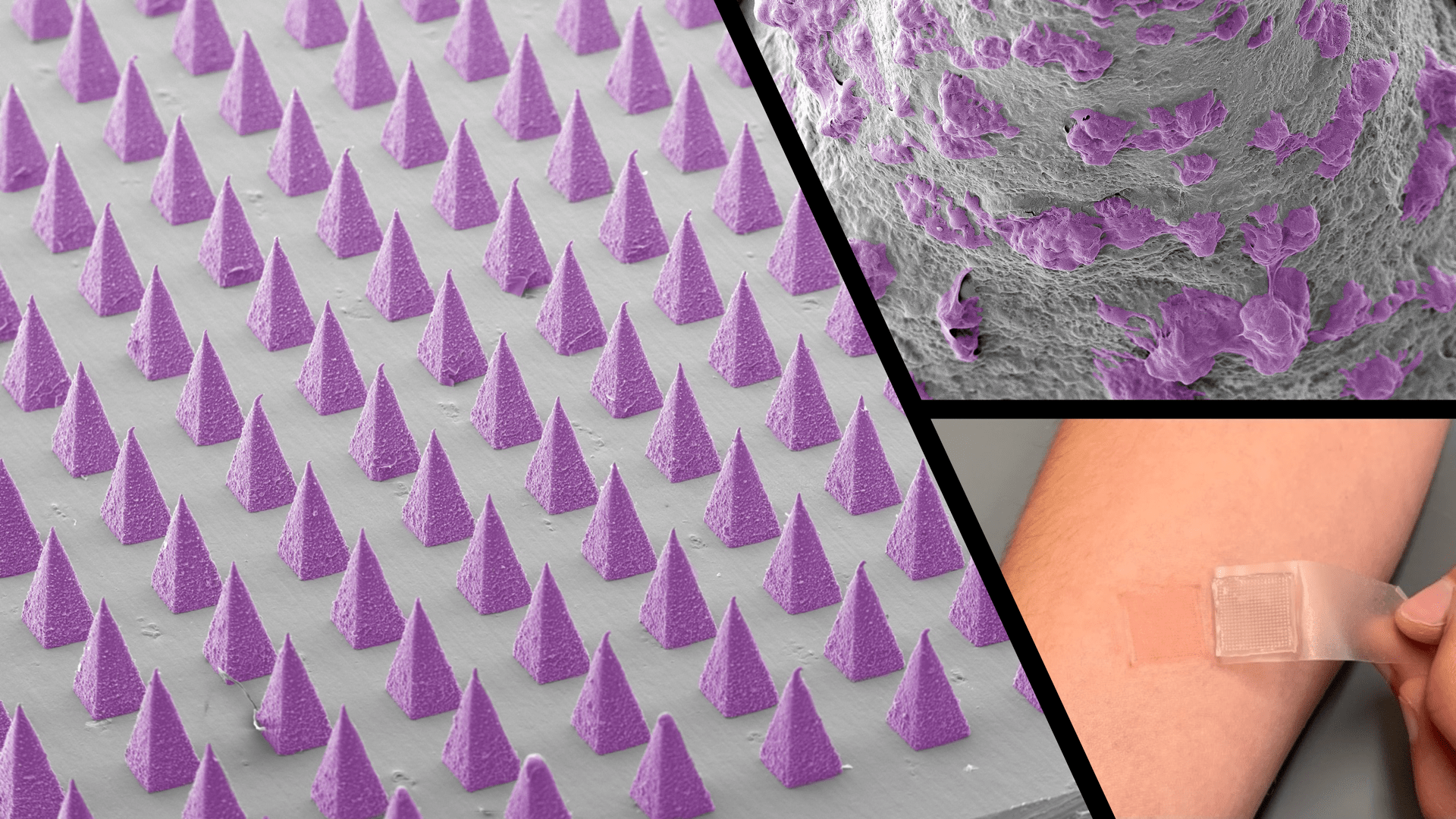
The skin represents a dynamic and complex ecosystem, harboring and interacting with a plethora of locally-entrenched commensal microorganisms. The skin microbiota induces protective and regulatory immunity that contributes to host-microbe mutualism. Skin dysbiosis has been associated with different inflammatory skin disorders, including atopic dermatitis and psoriasis. The Jalili laboratory is interested in using novel, noninvasive technologies, such as microneedle skin patches, to longitudinally monitor the host immune-microbiome interface at different stages of diseases without perturbing the sampling area. We integrate multi-omics data sets, including single-cell transcriptomics, metagenomics and proteomics, to elucidate how the skin microbiome and the immune system are cross-regulated in the inflammatory and autoimmune skin conditions.
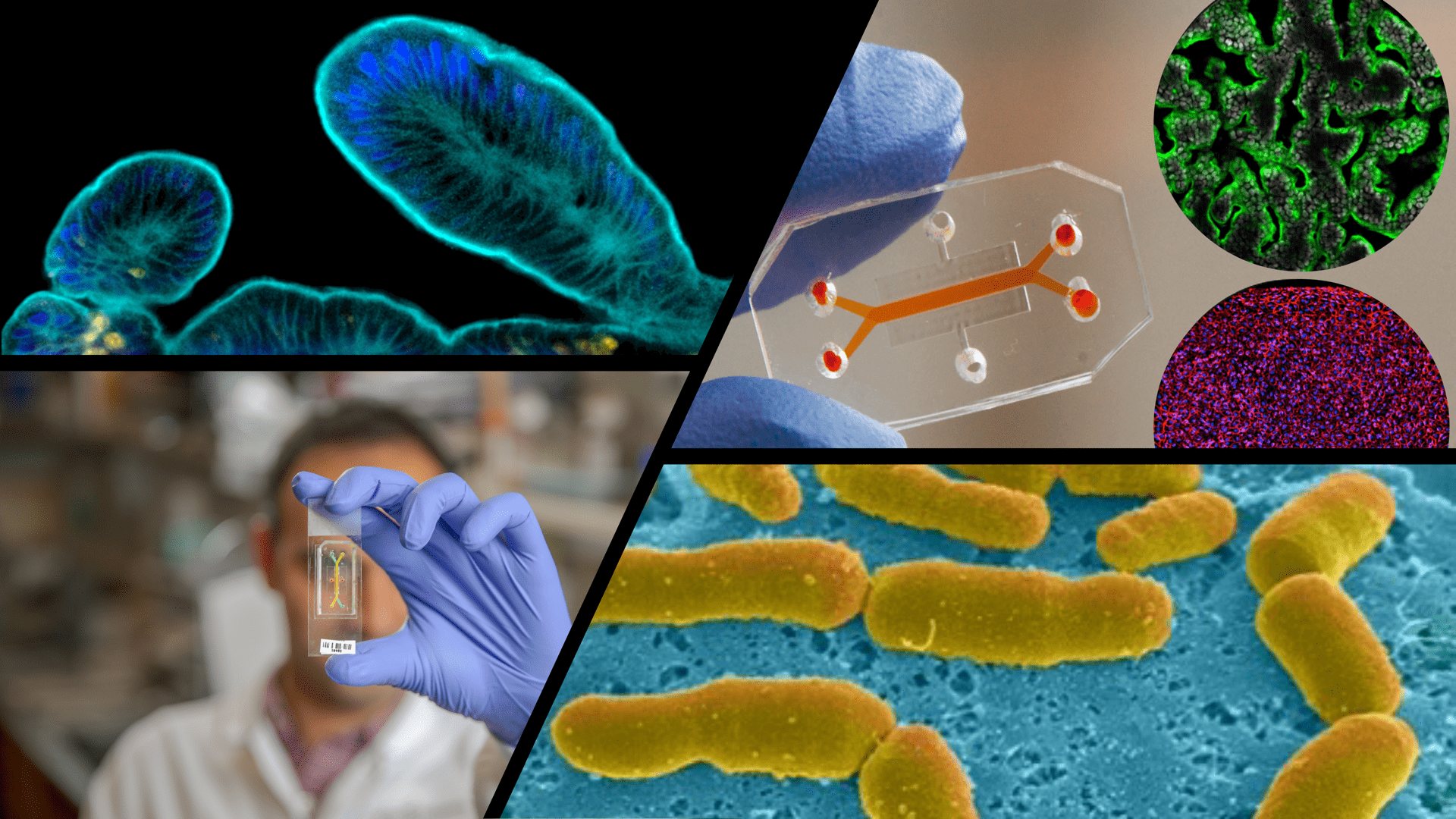
Bioinspired In vitro systems for disease modeling and therapeutic discovery
Although animal models have been used extensively to analyze host−microbiome interactions and their contributions to pathophysiology, there are limited in vitro systems available to recapitulate the complexity of the human organs and verify the host immune-microbiome interactions. Recent advances in tissue engineering, microfabrication and stem cell biology have enabled the development of more sophisticated systems, in particular organoid cultures and organ-on-chip models, that can provide unparalleled independent control over biomechanical, biochemical, and cellular parameters, and faithfully recapitulate the cellular microenvironment and tissue function and advance personalized medicine.
The available systems often lack the complex immune system and/or microbiome and are unable to faithfully mimic the long-term host-microbiome interactions that are essential in regulating number of autoimmune and infectious diseases. Thus, new systems are required to harbor both complex immune cells and microbiota to not only better model human diseases in vitro, but also serve as platforms for vaccine and therapeutic development. Our research group integrate animal and patient-derived organoids, iPSCs, immune cells and microbiome to develop complex models, such as engineered organoids and organ-on-a-chip systems, of human tissues and recapitulate the host-microbiome interactions in vitro. We are particularly interested in establishing inflammatory and autoimmune skin diseases on chip with the focus on psoriasis and dermatitis. We also model gastrointestinal immune-related diseases, with the specific focus on inflammatory bowel disease (IBD) and colon cancer.
Physiologically responsive synthetic and natural biomaterials
Simple and effective methods to deliver probiotics and microbiome-related therapeutics into the human tissues are still lacking and new biopolymer designs with tunable features are required to address this challenge. Our laboratory develops physiologically (e.g. pH and temperature) responsive biomaterials to enhance the delivery of microbiome-related therapeutics, prebiotics and nutraceuticals. We will also leverage these biomaterials to fabricate 3D scaffold and pursue bioprinting for soft tissue engineering and regenerative medicine purposes.
